Batteries made out of paper
The average home uses about 21 batteries every year, and then they throw them away. The United States throws away over 3 billion batteries, which is about one-fifth of the entire world's amount tossed into the garbage. "Garbage" means they end up mostly in landfills, which in itself is not good for the environment. The most common types of battery these days are alkaline or lithium. Alkaline types are made of steel and a mix of zinc/manganese/potassium/graphite, plus paper and plastic. Lithium batteries contain heavy metals (cobalt, copper and nickel) and organic solvents, and these can all leach into the ground and groundwater when the batteries corrode. Lithium batteries can also ignite and set landfills on fire! What if batteries were made of simple paper instead? Recent discoveries in science have shown this is becoming more of a commercial reality.
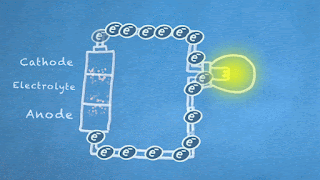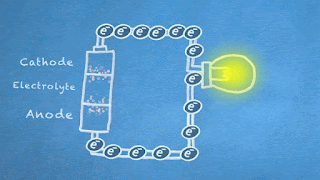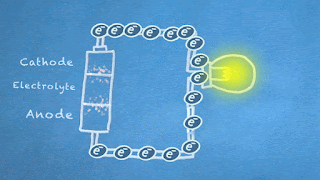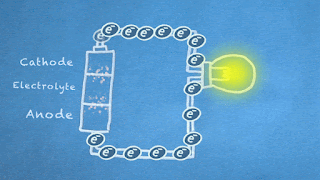
What are the basics of a battery anyway? It's a device with a positive pole (cathode) and a negative pole (anode) separated by a liquid (electrolyte) that makes a chemical reaction to generate electricity. Actually, the reaction happens between the electrolyte and the anode, and it produces electrons, which are negative in charge. Since negative charges repel each other, the electrons leave the anode and flow down a circuit if the anode and cathode are connected. On the way, they can do special jobs like light up a bulb before they return to the battery's cathode where they are attracted.
Earlier, in 2021, scientists from the NTU School of Electrical and Electronic Engineering developed a similar technology. They screen printed an ink layer of manganese on one side of a sheet of strengthened paper, and a layer of zinc and conductive carbon on the other. The paper was ordinary cellulose material that has been reinforced with hydrogel, a polymer that acts as the electrolyte. It also makes the end product very clear and flexible. The nearly transparent hydrogel paper lets people see exactly where the anode and cathode inks are when they are printed on. The picture below looks like they are printing the anode on top of the cathode, but that's just an optical illusion from the semi-transparent hydrogel paper. They used zinc for the anode and either nickel or manganese for the cathode.
The NTU paper battery was tested as a 4 cm x 4 cm (1.6 inch x 1.6 inch) sheet, and it powered a small fan for 45 minutes. What's more, as you can see below, the battery could be bent considerably without losing its capability to run the fan.
Not only is the hydrogel paper battery flexible, but it costs 10 times less than lithium type batteries to make. And, its materials are less harmful to the environment. The NTU researchers even tested it buried in soil on a rooftop garden exposed to nature. It was completely decomposed in a month.
They see several applications for these sorts of batteries.
- As a medical skin patch, it could warn the wearer to take insulin or asthma medication.
- As a GPS tracking device, it could be used on smaller items that current trackers can't attach to.
- As replacements for lithium batteries in smart watches, they could be used in different shapes and locations (like the wristband) and be lighter.
For now, there are down sides to overcome. The "just add water" type operates only when wet. The hydrogel paper type has a limited shelf life.
Here is a short (10-min) YouTube video showing how to make ink for paper batteries.
Here is a short YouTube video showing how to put carbon nanotube ink on paper to create a battery.






No comments:
Post a Comment