Meteorite impacts identified as driver of moon's tenuous atmosphere
Click on images to enlarge
The moon is made of green cheese. The moon has a dark side that never sees the light of the sun. There is no atmosphere on the moon. All of these are false, but that last one might come as a surprise, and it deserves closer investigation. Let's dive in and see what surrounds the Earth's moon.
Planets and their moons are formed from materials that make up their suns. Depending on their size, mass, and distance from the sun, they will end up with different exterior and interior structures, including atmospheres.
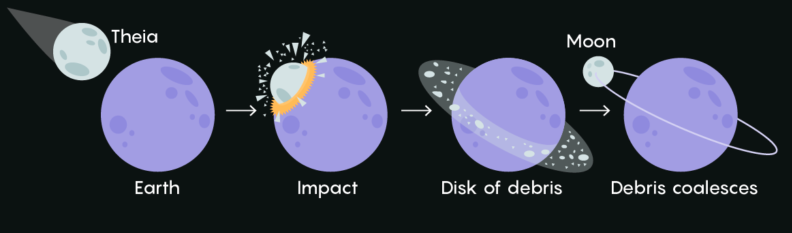
It is thought that the Earth's moon formed around the time that the Earth was still hot and molten, about 4.6 billion years ago. Something named Theia (the size of Mars) collided with Earth, and the debris that was ejected came together and formed the moon. So, the Earth's and moon's building block materials are the same. But why does Earth have such a thick atmosphere, and the moon doesn't?
Generally speaking, the stronger the gravitational pull, the thicker the atmosphere that a planet can hold near its surface. Over time, though, the sun's radiation blows away the lightweight elements like hydrogen and helium from the planets. Volcanic activity on the inner planets (Mercury, Venus, Earth, Mars) adds to the gases in their atmospheres. These events and more all combine to create a constantly changing mixture of gases. Here is what makes up the atmospheres of the 8 planets in our solar system.
As of August 4, 2024, the solar system has 293 known moons around its planets: Earth (1), Mars (2), Jupiter (95), Saturn (146), Uranus (28), Neptune (16), dwarf planet Pluto (5). Only about 15 have atmospheres (Saturn's moon Titan has the densest), and most of these barely have anything at all. They are called exospheres instead. The particles in an atmosphere are packed tightly enough to bounce off each other, but in an exosphere, the density is too thin, so they are considered "collision-less".
If the moon is just a piece of the ancient Earth, what has been found about gases surrounding it?
As far back at the 18th century, the Croatian scientist Roger Joseph Boscovich proposed there was no atmosphere there. He published a paper in 1753 with his results based on simple observations like:
- Stars suddenly disappear behind the moon instead of fade at its edge.
- There are no apparent clouds.
- There are no apparent instances of haze or winds blowing there.
An early hint that something might be there came from photos from the NASA Surveyor 7 lander in 1968. After sunset, a "glow" appeared on the horizon, which should not be seen if there is nothing above the surface to reflect or refract light. See the various pictures below.
Various Apollo missions also detected this lunar horizon glow. But by the early 1970s, it was determined to be refraction off moon dust kicked up as high as 10 km by infrared and ultraviolet rays from the sun stripping off electrons from moon dust and sending them above the surface. That's not an atmosphere. That's moon dust particles.
Three Apollo missions left behind instruments to try recording whatever thin atmosphere might be detected. Many of them gave results (argon-40, helium-4, oxygen, methane, nitrogen, carbon monoxide and carbon dioxide) that were hard to interpret because the moon lander and the instruments themselves gave off particles that interfered with the instruments. One, the Lunar Atmosphere Composition Experiment (LACE) presumably detected helium.
In 1985, sodium was found clinging just above the lunar surface, and then in 1989 potassium was found, too, both detected from instruments on Earth. If they were above the surface, this might explain why there is less of those two elements in lunar soil (regolith) than in Earth soil samples. They are being ripped off the lunar surface somehow.
Ten years later (2009), NASA sent the Lunar Reconnaisance Orbiter (LRO) specifically to examine the thin exosphere of the moon. It confirmed helium that had been detected by Apollo 17. Some comes from breakdown of thorium-232 and uranium-238 underground and released during moonquakes, but some also accumulates as part of the solar wind plasma (made of electrons, protons, and helium nuclei called alpha particles).
Shortly after that, the Lunar Atmosphere and Dust Environment Explorer (LADEE) spacecraft was sent by NASA in 2013. Its orbital measurements found helium-4, neon-20, and argon-40 elements in the thin exosphere.
Sputtering makes up <30% of released elements, while micrometeorites make up 70%. Gamma radiation also contributes a small fraction. LADEE did not learn this percentage, though; only a 2024 study by Nicole Nie in the Department of Earth, Atmospheric, and Planetary Sciences at the Massachusetts Institute of Technology determined that, over 50 years since the Apollo missions.
The known elements in the moon's exosphere are argon, helium, neon, potassium, and rubidium, plus small percentages of other elements. Sodium gets resorbed back into the lunar rock.
Computer simulation video of the origin of the moon. (NASA Ames Research Center)











No comments:
Post a Comment